COMBIGAN® HCP Indication and Important Safety Information
INDICATIONS AND USAGE:
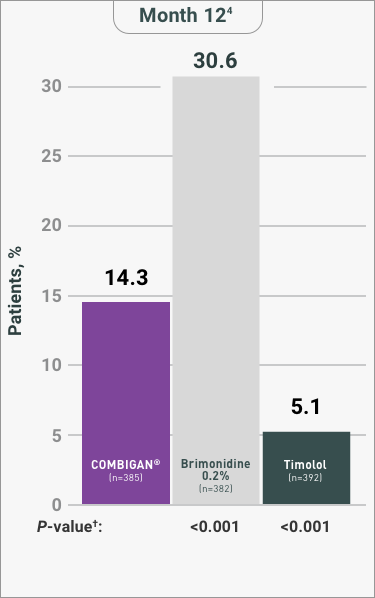
COMBIGAN® (brimonidine tartrate/timolol maleate ophthalmic solution)
0.2%/0.5% is an alpha-adrenergic receptor agonist with a beta-adrenergic receptor
inhibitor indicated for the
reduction of elevated intraocular pressure (IOP) in patients with glaucoma or ocular
hypertension who require
adjunctive or replacement therapy due to inadequately controlled IOP; the IOP-lowering
of COMBIGAN® dosed
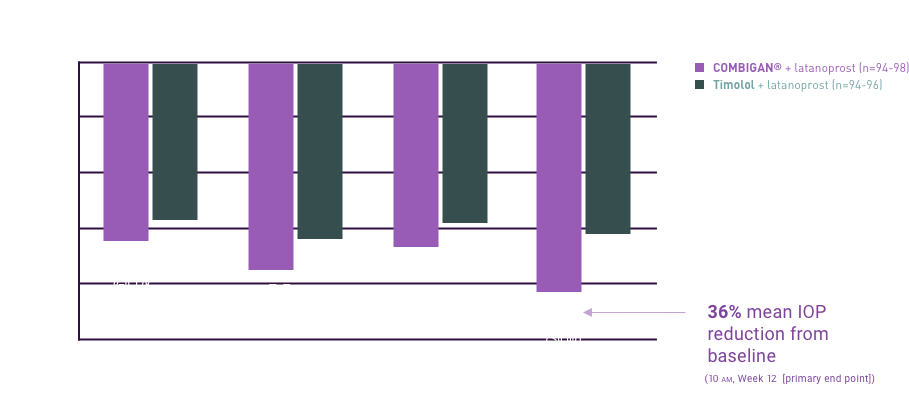
twice a day was slightly less than that seen with the concomitant administration of 0.5%
timolol maleate
ophthalmic solution dosed twice a day and 0.2% brimonidine tartrate ophthalmic solution
dosed three times per
day.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
COMBIGAN® is contraindicated in patients with reactive airway disease including
bronchial asthma; a history of bronchial asthma; severe chronic obstructive pulmonary
disease; in patients with
sinus bradycardia; second or third degree atrioventricular block; overt cardiac failure;
cardiogenic shock; in
neonates and infants (aged 2 years and younger); in patients with a hypersensitivity
reaction to any component
of COMBIGAN® in the past.
WARNINGS AND PRECAUTIONS:
COMBIGAN® contains timolol maleate. COMBIGAN® is administered
topically, but can be absorbed systemically. The adverse reactions with systemic
administration of beta-adrenergic
blocking agents may occur with topical use (eg, severe respiratory reactions and cardiac
reactions, including death
due to bronchospasm in patients with asthma, and rarely death in association with cardiac
failure, have been
reported with systemic or ophthalmic administration of timolol maleate). Ophthalmic
beta-blockers may impair
compensatory tachycardia and increase risk of hypotension.
Sympathetic stimulation may be essential to support the circulation in patients with
diminished myocardial
contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more
severe failure. In
patients with no history of cardiac failure, continued depression of the myocardium with
beta-blocking agents over
time can lead to cardiac failure. Discontinue COMBIGAN® at the first sign or
symptom of cardiac failure.
Patients with chronic obstructive pulmonary disease (eg, chronic bronchitis, emphysema) of
mild or moderate
severity, bronchospastic disease, or a history of bronchospastic disease should not receive
beta-blocking agents,
including COMBIGAN®.
COMBIGAN® may potentiate syndromes associated with vascular insufficiency. Use
caution in patients with
depression, cerebral or coronary insufficiency, Raynaud’s phenomenon, orthostatic
hypotension, or
thromboangiitis obliterans.
Patients taking beta-blockers with a history of atopy or severe anaphylactic reactions to a
variety of allergens may
be more reactive to repeated accidental, diagnostic, or therapeutic challenge with such
allergens. Such patients
may be unresponsive to the usual doses of epinephrine used to treat anaphylactic reactions.
Beta-adrenergic blockade can potentiate muscle weakness with myasthenic symptoms (eg,
diplopia, ptosis, and
generalized weakness). Although rare, timolol can increase muscle weakness in some patients
with myasthenia
gravis or myasthenic symptoms.
Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute
hypoglycemia and clinical
signs (eg, tachycardia) of hyperthyroidism. Use caution in patients subject to spontaneous
hypoglycemia or in
diabetic patients (especially those with labile diabetes) who are receiving insulin or oral
hypoglycemic agents.
Carefully manage patients who may develop thyrotoxicosis to avoid abrupt withdrawal of
beta-adrenergic blocking
agents that might precipitate a thyroid storm.
Ocular hypersensitivity has occurred with brimonidine tartrate ophthalmic solutions 0.2%
(eg, increase in IOP).
Some authorities recommend gradual withdrawal of beta-adrenergic receptor blocking agents
due to
impairment of beta-adrenergically mediated reflexes during surgery. If necessary during
surgery, the effects of
beta-adrenergic blocking agents may be reversed by sufficient doses of adrenergic agonists.
ADVERSE REACTIONS:
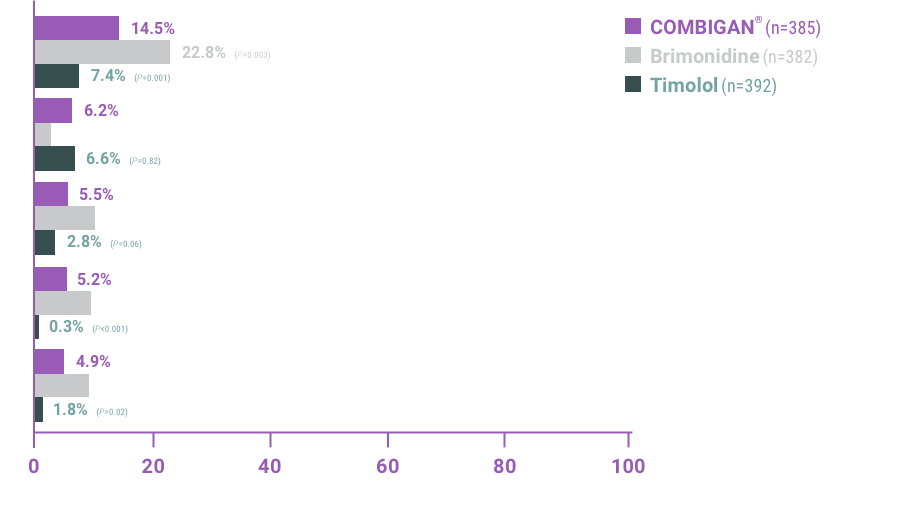
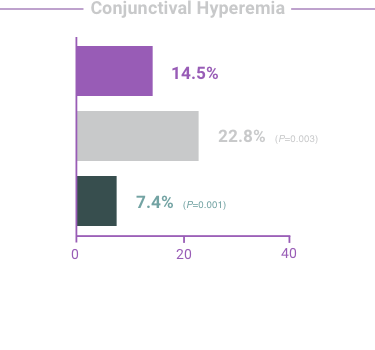
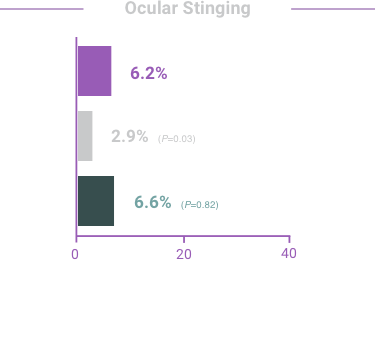
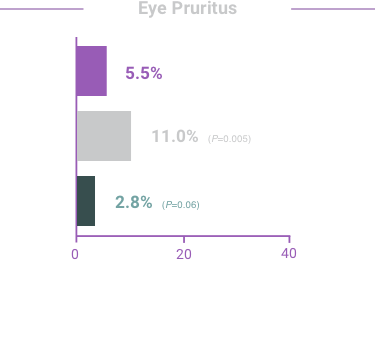
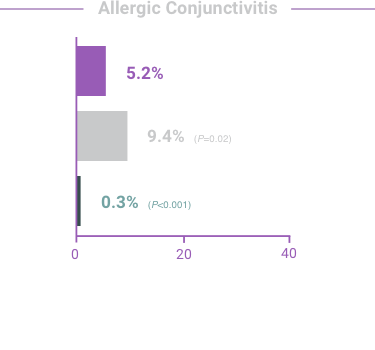
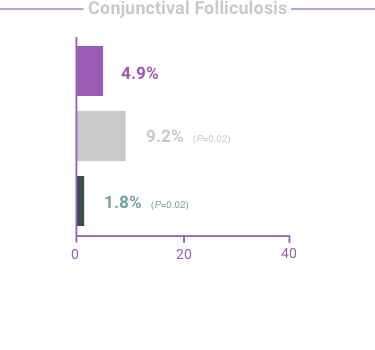
The most frequent reactions with COMBIGAN® in about 5% to 15% of patients
included: allergic conjunctivitis, conjunctival folliculosis, conjunctival hyperemia, eye
pruritus, ocular burning,
and stinging.
DRUG INTERACTIONS:
COMBIGAN® may reduce blood pressure. Use caution in patients on
antihypertensives and/or cardiac glycosides.
Observe patients receiving a beta-adrenergic blocking agent either orally or intravenously
and COMBIGAN® for
additive effects of beta-blockade, both systemic and on intraocular pressure. Concomitant
use of two topical
beta-adrenergic blocking agents is not recommended.
Use caution in the co-administration of beta-adrenergic blocking agents (eg,
COMBIGAN®) and oral or
intravenous calcium antagonists due to possible atrioventricular conduction disturbances,
left ventricular
failure, and hypotension. Avoid co-administration in patients with impaired cardiac
function.
Observe patients closely when a beta-blocker is administered to patients receiving
catecholamine-depleting
drugs (eg, reserpine) due to possible additive effects and the production of hypotension
and/or marked
bradycardia, which may result in vertigo, syncope, or postural hypotension.
Specific drug interaction studies have not been conducted with COMBIGAN®, but
consider the possibility of an
additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates,
sedatives, or anesthetics).
Concomitant use of beta-adrenergic blocking agents with digitalis and calcium antagonists
may have additive
effects in prolonging atrioventricular conduction time.
Potentiated systemic beta-blockade (eg, decreased heart rate, depression) has been reported
with combined
use of CYP2D6 inhibitors (eg, quinidine, SSRIs) and timolol.
Tricyclic antidepressants (TCAs) can blunt the hypotensive effect of systemic clonidine. It
is not known
whether the concurrent use of TCAs with COMBIGAN® in humans can interfere with
the IOP-lowering effect.
Caution is advised in patients taking TCAs, which can affect the metabolism and uptake of
circulating amines.
Monoamine oxidase (MAO) inhibitors may theoretically interfere with the metabolism of
brimonidine and
potentially increase systemic side effects such as hypotension. Use caution in patients
taking MAO inhibitors,
which can affect the metabolism and uptake of circulating amines.
Please see accompanying full Prescribing Information or visit https://www.rxabbvie.com/pdf/
combigan_pi.pdf