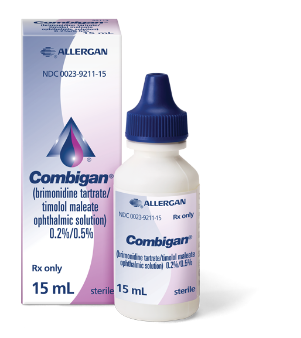
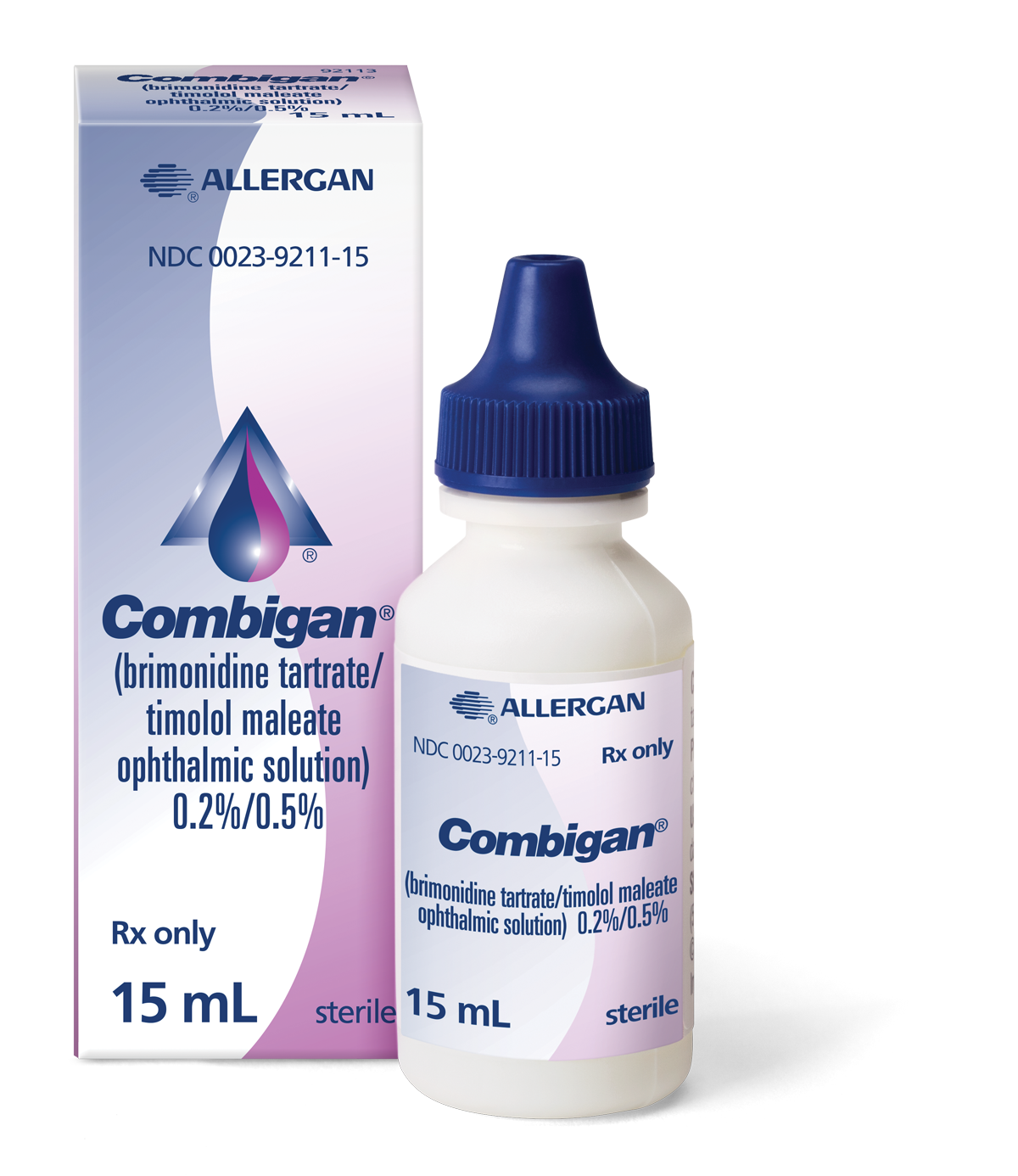
When it comes to the
pressure of your glaucoma
don’t compromise
See why COMBIGAN® may be the right additional IOP-lowering therapy for treating your open-angle glaucoma or ocular hypertension

IOP=intraocular pressure.
Eligible commercially-insured patients may pay as little as
$30* per 90-day prescription fill
That's as little as $10 a month for a 90-day supply
You can also enroll for discounts by
You can also enroll for discounts by
SAVINGS TO 72428†
*Maximum savings limits apply; patient out-of-pocket expense will vary depending on insurance coverage. Offer valid for patients with commercial prescription insurance coverage and a valid prescription for LUMIGAN® 0.01%, COMBIGAN®, or ALPHAGAN® P 0.1%. Offer not valid for patients enrolled in Medicare, Medicaid, or any other federal, state, or government-funded healthcare program. See At Your Service Savings Program Terms, Conditions, and Eligibility Criteria at SaveWithAYS.com.
†AYS Alerts: Msg and data rates apply. Msg frequency depends on user. Reply HELP for help; reply STOP to cancel. Consent to texts not required to sign up for offer. View our Mobile Terms & Conditions and Privacy Policy.
USE
COMBIGAN® (brimonidine tartrate/timolol maleate ophthalmic solution) 0.2%/0.5% is used for the reduction of high eye pressure, also called intraocular pressure (IOP), in patients with glaucoma who require additional (or adjunctive) IOP-lowering therapy. COMBIGAN® lowers IOP slightly less than taking both brimonidine tartrate 3 times a day and timolol maleate 2 times a day.
IMPORTANT SAFETY INFORMATION
Do not use COMBIGAN® 0.2%/0.5%:
- If you have reactive airway disease, including bronchial asthma, a history of bronchial asthma, or severe chronic obstructive pulmonary disease (COPD)
- If you have a very serious heart condition that causes a slowing of heart rate, such as sinus bradycardia (slow heart rate), second- or third-degree atrioventricular (AV) block, overt cardiac failure, or cardiogenic shock
- In neonates and infants (under the age of 2 years)
- If you are allergic or hypersensitive to any of the ingredients
- COMBIGAN® 0.2%/0.5% contains timolol maleate, a beta-blocker. With use of beta-blockers, there is a potential for severe respiratory or cardiac reactions (a range of conditions affecting the lungs or heart). These severe reactions may include death due to bronchospasm in patients with asthma and in patients with a history of heart failure and/or mild or moderate chronic bronchitis, emphysema, or similar lung conditions.
COMBIGAN® 0.2%/0.5% should be used with caution in patients with:
- Depression
- Cerebral or coronary insufficiency (conditions that result in reduced blood supply to the brain or heart)
- Raynaud’s phenomenon (a condition that results in reduced blood supply, most commonly, to the fingers and toes)
- Orthostatic hypotension (a form of low blood pressure that occurs when standing after sitting or lying down)
- Thromboangiitis obliterans (Buerger’s disease) (condition that results in reduced blood supply to the arms and legs, usually presenting in fingers and feet)
- A propensity for or a history of: severe allergic reactions, including anaphylactic reactions to a variety of allergens
- New or worsening muscle weakness and/or other symptoms of myasthenia gravis (eyelid droop, double vision, and generalized weakness)
- Diabetes and spontaneous low blood sugar; the symptoms of sudden low blood sugar may be masked
- Hyperthyroidism
Avoid allowing the tip of the dispensing bottle to touch the eye, anything around the eye, fingers, or any other surface to avoid contamination by common bacteria known to cause eye infections (bacterial keratitis). Using contaminated solutions can cause serious damage to the eye and loss of vision. Always replace the cap after using. Do not use after the expiration date marked on the bottle or if the solution changes color or becomes cloudy.
If you have eye surgery, eye trauma or infection, or develop any eye reactions, immediately consult with your physician about continuing the use of COMBIGAN® 0.2%/0.5%.
If you use more than one drug in the eye, be sure to wait at least 5 minutes between each drug application.
COMBIGAN® 0.2%/0.5% contains an ingredient that may be absorbed by and cause discoloration of soft contact lenses. If you wear contact lenses, remove them before using COMBIGAN® 0.2%/0.5%. Then wait 15 minutes after using COMBIGAN® 0.2%/0.5% before you put your contacts back into your eyes.
As with other similar medications, COMBIGAN® 0.2%/0.5% may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be aware of the potential for a decrease in mental alertness.
The most common side effects (5%-15%) in the clinical trial include allergic eye reaction, inflammation of the conjunctiva (“pink eye”), eye redness, itchy eye, eye burning, and stinging sensations. These are not all of the possible side effects with COMBIGAN® 0.2%/0.5%. Tell your doctor if you have any side effect that bothers you or that does not go away.
Please see full Prescribing Information or visit
https://www.rxabbvie.com/pdf/
combigan_pi.pdf.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/myAbbVieAssist to learn more.